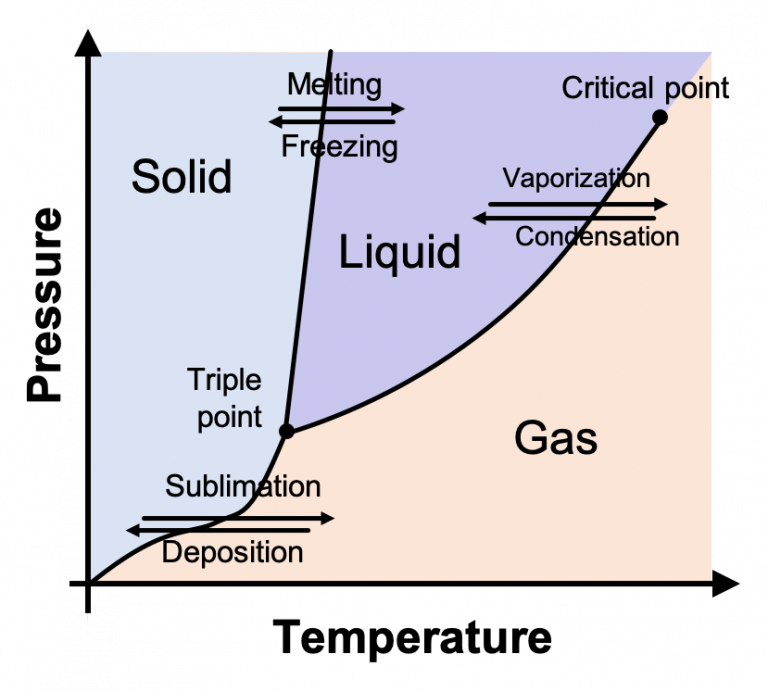
Pressure Temperature Chart Solid Liquid Gas . with most substances, the temperature and pressure related to the triple point lie below standard temperature and. the three phases coexist at a single pressure and temperature. This is known as the triple point and is described. a phase diagram shows the temperatures and pressures at which the various phases (i.e., solid, liquid and gas) of a substance. At low pressure and high temperature, the. The gas phase is favored at high temperature and low pressure. the solid phase is favored at low temperature and high pressure; at high pressures and low temperatures, the substance is in the solid phase.
from partdiagramtexanisch03.z13.web.core.windows.net
At low pressure and high temperature, the. with most substances, the temperature and pressure related to the triple point lie below standard temperature and. This is known as the triple point and is described. at high pressures and low temperatures, the substance is in the solid phase. a phase diagram shows the temperatures and pressures at which the various phases (i.e., solid, liquid and gas) of a substance. the three phases coexist at a single pressure and temperature. The gas phase is favored at high temperature and low pressure. the solid phase is favored at low temperature and high pressure;
Phase Diagram Pressure And Temperature
Pressure Temperature Chart Solid Liquid Gas The gas phase is favored at high temperature and low pressure. This is known as the triple point and is described. with most substances, the temperature and pressure related to the triple point lie below standard temperature and. The gas phase is favored at high temperature and low pressure. At low pressure and high temperature, the. the three phases coexist at a single pressure and temperature. at high pressures and low temperatures, the substance is in the solid phase. a phase diagram shows the temperatures and pressures at which the various phases (i.e., solid, liquid and gas) of a substance. the solid phase is favored at low temperature and high pressure;
From www.rachelatalldrinkofwater.com
Solids, Liquids, & Gases! Rachel A Tall Drink of Water Pressure Temperature Chart Solid Liquid Gas the three phases coexist at a single pressure and temperature. At low pressure and high temperature, the. The gas phase is favored at high temperature and low pressure. with most substances, the temperature and pressure related to the triple point lie below standard temperature and. a phase diagram shows the temperatures and pressures at which the various. Pressure Temperature Chart Solid Liquid Gas.
From www.sexiezpicz.com
Ammonia Pressure Temperature Chart SexiezPicz Porn Pressure Temperature Chart Solid Liquid Gas a phase diagram shows the temperatures and pressures at which the various phases (i.e., solid, liquid and gas) of a substance. This is known as the triple point and is described. At low pressure and high temperature, the. the three phases coexist at a single pressure and temperature. at high pressures and low temperatures, the substance is. Pressure Temperature Chart Solid Liquid Gas.
From ricksfreeautorepairadvice.com
Diagnose Car AC problems — step by step approach — Ricks Free Auto Pressure Temperature Chart Solid Liquid Gas At low pressure and high temperature, the. with most substances, the temperature and pressure related to the triple point lie below standard temperature and. the solid phase is favored at low temperature and high pressure; at high pressures and low temperatures, the substance is in the solid phase. This is known as the triple point and is. Pressure Temperature Chart Solid Liquid Gas.
From repairfixindijanacaq.z22.web.core.windows.net
How To Read R410a Pressure Chart Pressure Temperature Chart Solid Liquid Gas the three phases coexist at a single pressure and temperature. at high pressures and low temperatures, the substance is in the solid phase. At low pressure and high temperature, the. This is known as the triple point and is described. with most substances, the temperature and pressure related to the triple point lie below standard temperature and.. Pressure Temperature Chart Solid Liquid Gas.
From www.myxxgirl.com
Anhydrous Ammonia Pressure Temperature Chart My XXX Hot Girl Pressure Temperature Chart Solid Liquid Gas At low pressure and high temperature, the. the solid phase is favored at low temperature and high pressure; This is known as the triple point and is described. a phase diagram shows the temperatures and pressures at which the various phases (i.e., solid, liquid and gas) of a substance. the three phases coexist at a single pressure. Pressure Temperature Chart Solid Liquid Gas.
From repairmachineletsbeilshe.z4.web.core.windows.net
R410 Pressure Temp Chart Pressure Temperature Chart Solid Liquid Gas with most substances, the temperature and pressure related to the triple point lie below standard temperature and. The gas phase is favored at high temperature and low pressure. the solid phase is favored at low temperature and high pressure; the three phases coexist at a single pressure and temperature. at high pressures and low temperatures, the. Pressure Temperature Chart Solid Liquid Gas.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps Pressure Temperature Chart Solid Liquid Gas at high pressures and low temperatures, the substance is in the solid phase. The gas phase is favored at high temperature and low pressure. the solid phase is favored at low temperature and high pressure; This is known as the triple point and is described. the three phases coexist at a single pressure and temperature. At low. Pressure Temperature Chart Solid Liquid Gas.
From rebeccahdougie.blogspot.com
RebeccahDougie Pressure Temperature Chart Solid Liquid Gas with most substances, the temperature and pressure related to the triple point lie below standard temperature and. a phase diagram shows the temperatures and pressures at which the various phases (i.e., solid, liquid and gas) of a substance. At low pressure and high temperature, the. the solid phase is favored at low temperature and high pressure; . Pressure Temperature Chart Solid Liquid Gas.
From edwardr777.github.io
R22 Temperature Pressure Chart Pressure Temperature Chart Solid Liquid Gas a phase diagram shows the temperatures and pressures at which the various phases (i.e., solid, liquid and gas) of a substance. at high pressures and low temperatures, the substance is in the solid phase. with most substances, the temperature and pressure related to the triple point lie below standard temperature and. The gas phase is favored at. Pressure Temperature Chart Solid Liquid Gas.
From workshopfixpiroutteh3.z22.web.core.windows.net
Temperature Pressure Chart 410a Pressure Temperature Chart Solid Liquid Gas a phase diagram shows the temperatures and pressures at which the various phases (i.e., solid, liquid and gas) of a substance. the three phases coexist at a single pressure and temperature. This is known as the triple point and is described. at high pressures and low temperatures, the substance is in the solid phase. the solid. Pressure Temperature Chart Solid Liquid Gas.
From repairfixgaroares.z13.web.core.windows.net
R410a Pressure Chart High And Low Side Pdf Pressure Temperature Chart Solid Liquid Gas The gas phase is favored at high temperature and low pressure. the three phases coexist at a single pressure and temperature. at high pressures and low temperatures, the substance is in the solid phase. a phase diagram shows the temperatures and pressures at which the various phases (i.e., solid, liquid and gas) of a substance. At low. Pressure Temperature Chart Solid Liquid Gas.
From schematicdatabitos99.z22.web.core.windows.net
Liquid Liquid Phase Diagram Pressure Temperature Chart Solid Liquid Gas at high pressures and low temperatures, the substance is in the solid phase. the three phases coexist at a single pressure and temperature. At low pressure and high temperature, the. the solid phase is favored at low temperature and high pressure; The gas phase is favored at high temperature and low pressure. a phase diagram shows. Pressure Temperature Chart Solid Liquid Gas.
From diagramdataenergies.z14.web.core.windows.net
How To Interpret A Phase Change Diagram Pressure Temperature Chart Solid Liquid Gas at high pressures and low temperatures, the substance is in the solid phase. The gas phase is favored at high temperature and low pressure. At low pressure and high temperature, the. the solid phase is favored at low temperature and high pressure; with most substances, the temperature and pressure related to the triple point lie below standard. Pressure Temperature Chart Solid Liquid Gas.
From www.mastercool.com
Mastercool Inc., Manufacturer of Air Conditioning, Refrigeration Pressure Temperature Chart Solid Liquid Gas At low pressure and high temperature, the. the three phases coexist at a single pressure and temperature. This is known as the triple point and is described. at high pressures and low temperatures, the substance is in the solid phase. with most substances, the temperature and pressure related to the triple point lie below standard temperature and.. Pressure Temperature Chart Solid Liquid Gas.
From grassrootsmotorsports.com
A/C diagnosis high side low, low side high Grassroots Motorsports Pressure Temperature Chart Solid Liquid Gas a phase diagram shows the temperatures and pressures at which the various phases (i.e., solid, liquid and gas) of a substance. the three phases coexist at a single pressure and temperature. with most substances, the temperature and pressure related to the triple point lie below standard temperature and. at high pressures and low temperatures, the substance. Pressure Temperature Chart Solid Liquid Gas.
From partdiagramtexanisch03.z13.web.core.windows.net
Phase Diagram Pressure And Temperature Pressure Temperature Chart Solid Liquid Gas at high pressures and low temperatures, the substance is in the solid phase. the three phases coexist at a single pressure and temperature. At low pressure and high temperature, the. a phase diagram shows the temperatures and pressures at which the various phases (i.e., solid, liquid and gas) of a substance. the solid phase is favored. Pressure Temperature Chart Solid Liquid Gas.
From dxohhontq.blob.core.windows.net
What Pressure Does Co2 Liquify at Kathryn Rowan blog Pressure Temperature Chart Solid Liquid Gas the solid phase is favored at low temperature and high pressure; at high pressures and low temperatures, the substance is in the solid phase. with most substances, the temperature and pressure related to the triple point lie below standard temperature and. a phase diagram shows the temperatures and pressures at which the various phases (i.e., solid,. Pressure Temperature Chart Solid Liquid Gas.
From www.410achiller.com
Temperature Pressure Chart R134A R407C R404A R410A Pressure Temperature Chart Solid Liquid Gas This is known as the triple point and is described. The gas phase is favored at high temperature and low pressure. At low pressure and high temperature, the. at high pressures and low temperatures, the substance is in the solid phase. the three phases coexist at a single pressure and temperature. with most substances, the temperature and. Pressure Temperature Chart Solid Liquid Gas.